~Why do polar solvents swell urethane squeegees?~
In a previous column, I wrote that polar solvents swell urethane squeegees violently.
This time I would like to explain a little more about polar solvents.
This time I would like to explain a little more about polar solvents.
First, what is a polar solvent?
As I wrote in this column before, "polarity" is the electrical bias within a molecule.
Solvents used in ink that have a large electrical bias are called "polar solvents."Typical examples are water and alcohol.
Solvents used in ink that have a large electrical bias are called "polar solvents."Typical examples are water and alcohol.
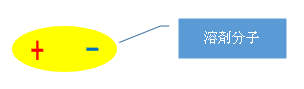
What is electrical bias?
Atoms exist around the nucleus, but the power to attract electrons differs depending on the substance.
Oxygen atoms [O], nitrogen atoms [N], and chlorine atoms [Cl], which are closely related to screen printing solvents, are atoms with a strong force that attracts electrons.Electrons are attracted to these atoms in the solvent (substance) they are in.
And if these atoms are unevenly bonded in the solvent molecule, the part of the solvent molecule that attracts electrons will be negative, and the other side will be positive. This is "polarity", which is electrical bias.
Atoms exist around the nucleus, but the power to attract electrons differs depending on the substance.
Oxygen atoms [O], nitrogen atoms [N], and chlorine atoms [Cl], which are closely related to screen printing solvents, are atoms with a strong force that attracts electrons.Electrons are attracted to these atoms in the solvent (substance) they are in.
And if these atoms are unevenly bonded in the solvent molecule, the part of the solvent molecule that attracts electrons will be negative, and the other side will be positive. This is "polarity", which is electrical bias.
The molecular structure of the polar solvent often used in screen printing is as follows.
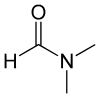
*N,N-dimethylformamide
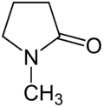
NMP (N-methylpyrrolidone)
There are oxygen atoms [O] and nitrogen atoms [N] in the molecule. Moreover, these atoms are biased. Therefore, it is a highly polar solvent.
So why do polar solvents swell urethane squeegees?
In the previous column, I explained that the "urethane bond", which is the center of the chemical bond of ester-based urethane rubber, has "polarity". This molecular structure is shown below.
In the previous column, I explained that the "urethane bond", which is the center of the chemical bond of ester-based urethane rubber, has "polarity". This molecular structure is shown below.
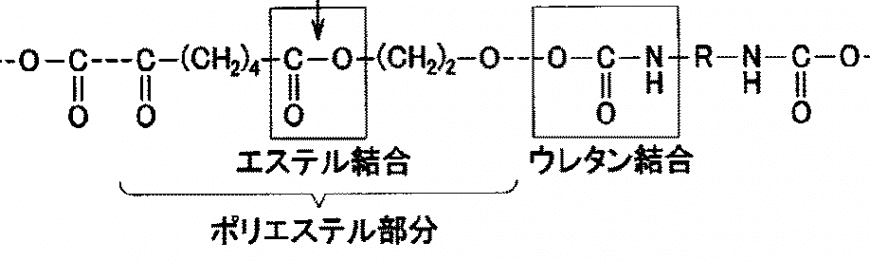
Look at the part that says "ester bond".
There is an oxygen atom [O] in the molecule and it is biased. Therefore, ester-based urethane rubber (urethane squeegee) also has polarity.
When a polar solvent and a polar urethane come into contact with each other, the solvent and urethane attract and swell, just like magnets stick to each other.
Next, the molecular structure of non-polar solvents often used in screen printing is as follows.
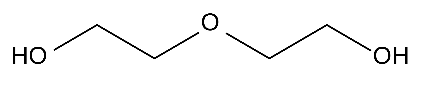
Diethylene glycol
There is an oxygen atom [O] in the molecule, but it is in the center of the molecule and is not biased, so it has no polarity.
If such a solvent is used, it will not swell easily because it will not attract urethane.
If such a solvent is used, it will not swell easily because it will not attract urethane.
Non-polar solvents are often used for the ink used in screen printing. Urethane rubber has excellent abrasion resistance, so urethane squeegees are fine for most printing applications, but the only drawback is swelling caused by polar solvents.
